american academy of pediatrics covid vaccine recommendations
ACIP recommends that adults aged 65 years preferentially receive one of the following influenza vaccines. Since COVID-19 vaccines were recommended for everyone age 5 and up millions of children and teens have been safely vaccinated.
Now to keep as many kids protected as possible a booster.

. CDC and the American Academy of Pediatrics AAP recommend children catch up on routine childhood vaccinations following disruptions from COVID-19. No product is preferred over. The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents.
Children 6 through 35 months of age may receive any licensed age-appropriate IIV available this season at the dose indicated for the vaccine. Quadrivalent high-dose inactivated influenza vaccine HD-IIV4. Children 6 months through 8 years of age who are receiving influenza vaccine for the first time or who received only 1 dose before July 1 2022 or whose vaccination status is.
Children ages 5-11 years are now eligible for a bivalent COVID-19 vaccine booster. You have the power. The American Academy of Pediatrics AAP recommends coronavirus disease 2019 COVID-19 vaccination for all infants children and adolescents 6 months of age and.
Today the Advisory Committee on Immunization Practices ACIP of the Centers for Disease Control and Prevention CDC recommended two COVID-19 vaccines. FDA panel recommends Moderna COVID vaccines for children ages 6-17 June 14 2022 -- If FDA and CDC leaders sign off later this week parents would have a second vaccine. The COVID-19 vaccine is our best hope for ending the current pandemic.
Below are common questions and answers. The nearly 4 million children diagnosed with COVID-19 so far account for 14 of cases according to a recent report by the American Academy of Pediatrics AAP and the. American Academy of Pediatrics AmerAcadPeds October 19 2022 Equitable access to COVID-19 vaccines for all ages and populations remains critically.
Leaders of the Food and Drug Administration FDA and Centers for Disease Control and. COVID-19 vaccine is currently authorized for some adolescents and will likely be authorized for adolescents 12 years of age and older soon. The American Academy of Pediatrics AAP in collaboration with the Centers for Disease Control and Prevention CDC conducted 9 key informant interviews with emerging pediatric practices.
American Academy of Pediatrics Guidance. American Academy of Pediatrics Offers Guidance for Caring and Treatment of Long-Term Cancer Survivors. While the goal will be to administer vaccine to as many people as possible as quickly as possible vaccine supply will.
Pediatricians are eager to help protect children and families by administering the vaccine the most effective prevention tool available since the start of the pandemic American Academy of. Learn more about policy recommendations and the regulations and laws that the. ITASCA ILThe American Academy of Pediatrics supports todays recommendation by the Advisory Committee on Immunization Practices ACIP of the Centers.
The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents. The sooner younger children can be included in research the better as widespread vaccinations will help protect communities. The AAP recommends COVID-19 vaccination for all children and adolescents 6 months of age and older who do not have contraindications using a vaccine authorized for use.

Resources To Promote Covid 19 Vaccines For Children Teens Cdc
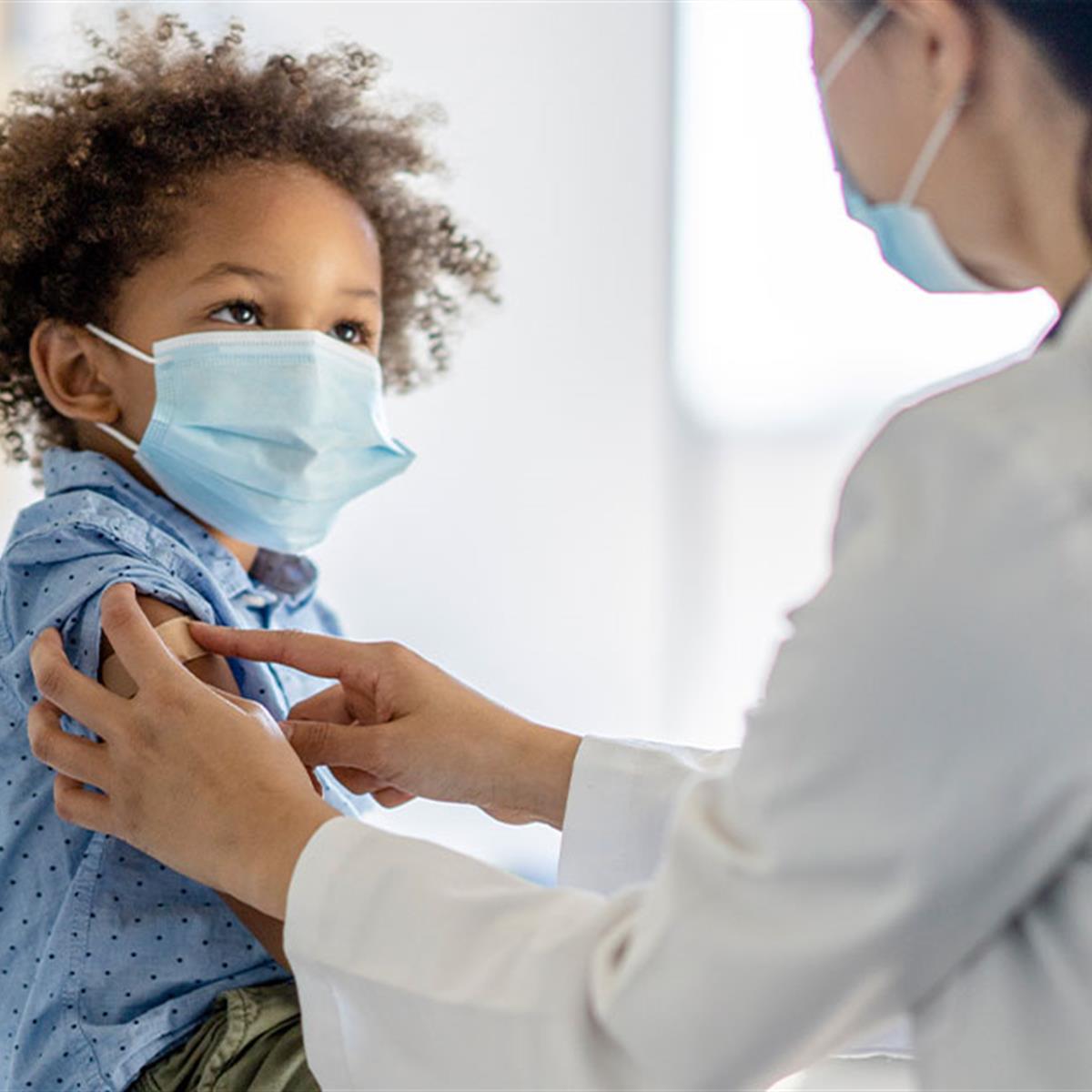
What Should Parents Know About The Covid Vaccine For Kids Under 5 Healthychildren Org

Covid 19 Vaccine For 12yo And Up Alliance Pediatrics

From Unmc Nebraska Med Covid 19 Vaccine For Kids Under Five Now Available Noise

Covid 19 Vaccine Uptake Toolkit Alabama Chapter Of The American Academy Of Pediatrics
Covid 19 Vaccine And Children Chester County Pa Official Website
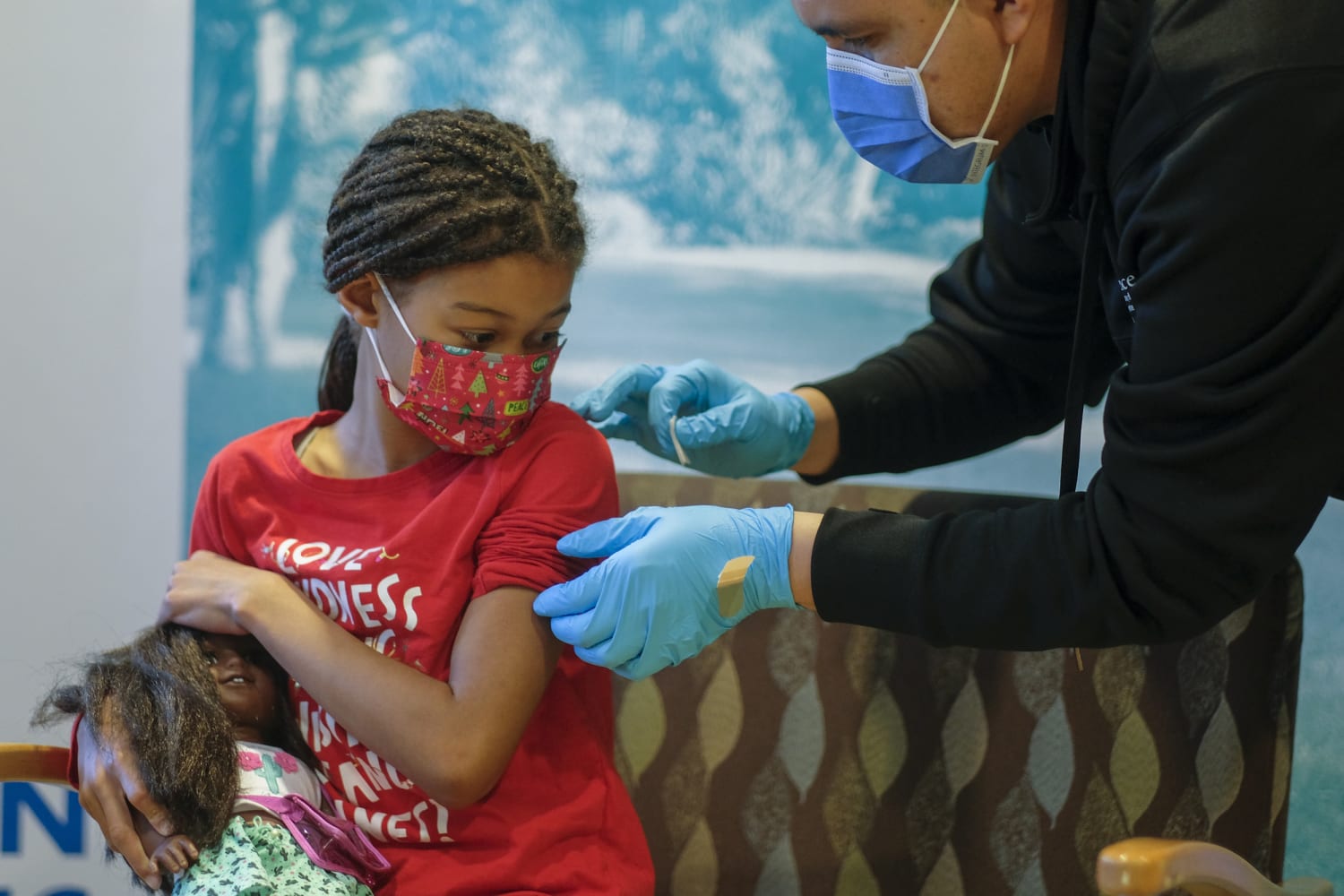
Kid S Covid Vaccine Cdc Group Says Add Vaccine To Routine Immunization Schedule
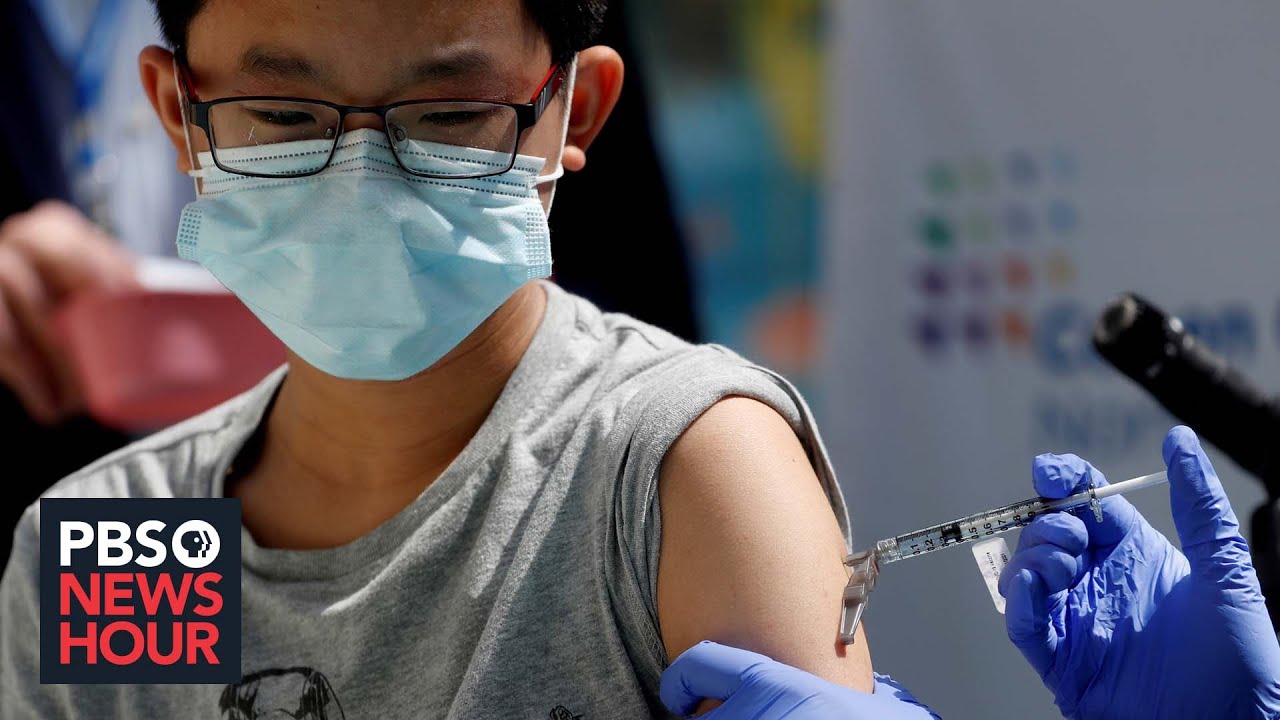
American Academy Of Pediatrics Urges Fda To Approve Covid Vaccines For Children Under 12 Pbs Newshour

Pediatric Covid 19 Vaccine Dosing Quick Reference Guide Wiaap

Upcoming Covid 19 Vaccine Clinics Lexington Fayette County Health Department
Coronavirus Vaccine Orange County Nc

American Academy Of Pediatrics The American Academy Of Pediatrics Applauds The Cdc Advisory Committee S Approval Of A Safe Effective Covid 19 Vaccine In Children Ages 5 11 Read The Full Statement Here Http Ow Ly S9vg50gemcl

Covid 19 Information And Resources Tnaap

Cdphe Recommends Parents And Guardians Plan For Covid 19 Vaccines For 5 11 Year Olds Colorado Covid 19 Updates

Covid Children As Young As 6 Months Now Eligible For Pfizer Moderna Vaccines

